-

-

-

- HOME
- 연구동향
- MERRIC인
- 메릭웨비나
- 공학DB
- 문헌정보
- 기계로봇소식
- MERRIC은?
- 회원가입
- 로그인
- 물성치테이블
- 용어사전
- 실험실소개
| 지도교수 | 유재석 |
|---|---|
| 전공분류 | 기타(ETC) |
| 주소 | 대구광역시 달성군 현풍면 테크노중앙대로 333 |
| 전화 | |
| 홈페이지 | http://ultrasound.dgist.ac.kr/ |
The Advanced Ultrasound Research Laboratory was established in Dec. 2019 within the Department of Robotics Engineering at DGIST. Our group is dedicated to development of a revolutionary biomedical imaging and therapeutic techniques based on ultrasound to solve many complementary challenges in health-care. Our research scope covers broad interests: (1) from the visualization of anatomical information to the assessment of functional features, (2) from cellular to organ level imaging, and (3) from diagnostic imaging to theranositcs. Our convergence research across the full spectrum of fields is at the forefront of the discovery of the fundamental science, development of state-of-the-art imaging technologies and systems, and clinical translation of biomedical imaging technologies.
OVERALL
Based on the understanding of fundamental wave physics, a synergistic combination of multi-modal ultrasound and photoacoustic imaging is utilized to derive innovative solutions to complementary challenging problems in biomedical imaging, image-guided therapy, and bio-nanotechnology. The state-of-the-art hybrid imaging system will provide a powerful platform for translational theranostics (therapeutics + diagnostics) research towards a clinical utility.
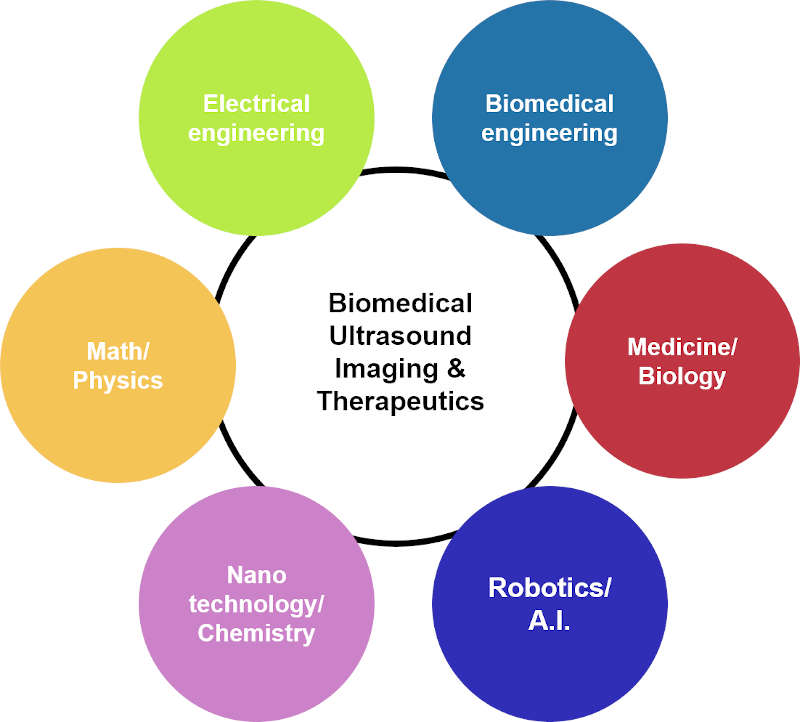
FIG. Convergence research approaches
BIOMEDICAL MULTI-MODAL ULTRASOUND IMAGING
Biomedical multi-modal imaging approach has significant potential in early diagnosis, therapy management, and monitoring of therapeutic outcome for cancer, cardiovascular disease and other pathologies by providing comprehensive disease information. With sharply increasing demands of functional imaging capability, several ultrasound imaging technologies have been developed to provide functional features of tissue, such as (1) elastography to assess tissue mechanical property (Elasticity), (2) thermal strain imaging to characterize tissue compositional property (Water-based tissue vs Fatty tissue), (3) Doppler imaging to evaluate hemodynamics and (4) contrast-enhanced ultrasound imaging using microbubbles to assess blood perfusion, in addition to anatomical information provided by (5) traditional grey-scale sonography. To further foster typical ultrasound imaging, in recent years (6) photoacoustic imaging that can detect optical contrast at an ultrasonic deep imaging depth has been developed. One of our interests was the implementation of the multi-modal imaging system; combining B-mode (traditional sonography), ultrasound elastography, thermal strain imaging, and photoacoustic imaging into the single platform to provide multiple information; anatomical, mechanical, compositional, and optical information on the same imaging plane, simultaneously. With the developed imaging platform for preclinical and clinical research, advanced imaging technologies will be further developed for the extension of imaging capabilities.
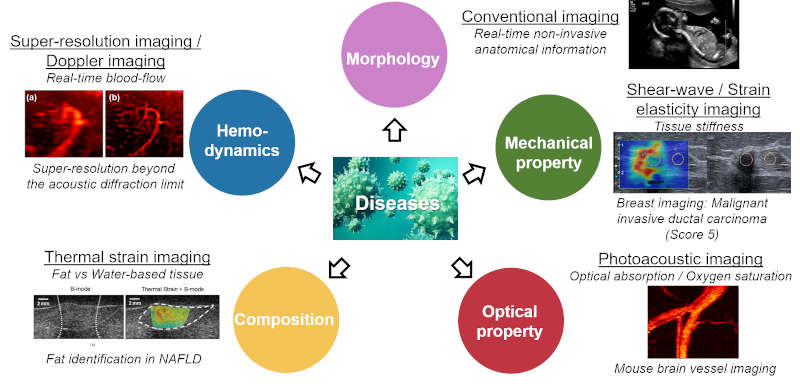
FIG. Multi-modal ultrasound imaging technologies
SUPER-RESOLUTION ULTRASOUND IMAGING
Ultrasound imaging has the advantage of safety, noninvasiveness, portability, affordability, and ease of use. Several approaches, such as Doppler ultrasound imaging and contrast-enhanced ultrasound imaging have been explored to diagnose diseases changing microvasculature such as progression from acute kidney injury to chronic kidney disease or vasa vasorum development of atherosclerosis. However, neither technique provides spatial resolution high enough for assessing microvessels, especially in the cortex. This is mainly because of the insufficient sensitivity and the acoustic diffraction limit of the operating ultrasound frequency. Super-resolution ultrasound imaging is an emerging technology that can achieve a high spatial resolution of vasculature beyond the acoustic diffraction limit. The unprecedented spatial resolution is accomplished by combining the use of ultrasound contrast agents that enhance hyperechoic contrast, ultrafast frame rate imaging, advanced clutter filtering technique that extracts signals only from microbubbles circulating in the vessels, and novel center localization techniques that pinpoint the center of each microbubble from the extracted signals. Thus, the individual microvessels can be identified with unmatched spatial resolution up to one fifth of the wavelength of the ultrasound waves, significantly outperforming conventional ultrasound approaches.
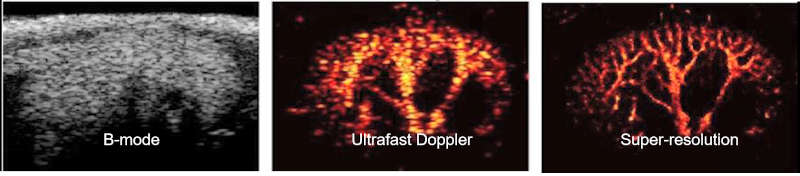
FIG. Imaging on mouse kidney: B-mode, Ultrafast Doppler, Super-resolution imaging (x5 better resolution)
3-D ULTRASOUND IMAGING
Volumetric ultrasound, a promising biomedical diagnostic imaging tool, enables acquisition of three-dimensional, multi-planar visualization of tissue for more accurate and objective diagnosis, improved guidance of surgical intervention, and therapy monitoring. Modern volumetric ultrasound images can be acquired in two methods: mechanical and electrical scanning. Mechanical scanning with a bulky motorized 1-D array transducer is not fast enough in scanning speed to avoid potential motion artifacts. Furthermore, mechanical scanning cannot produce C-plane (coronal plane, i.e., images of the plane orthogonal to the ultrasound beam direction) images in real-time because full volumetric information is accessible only upon completion of an entire scanning session. In contrast, electronic scanning using a fully-sampled matrix array can acquire full volume information without mechanical sweeping, enabling high frame rate multi-planar imaging. Ultrafast volumetric imaging using a 1024-element matrix array has been recently introduced to implement 3-D shear wave elasticity imaging and 3-D Doppler imaging techniques. We will further investigate the novel volumetric imaging technique based on the matrix array and the row-column matrix array.
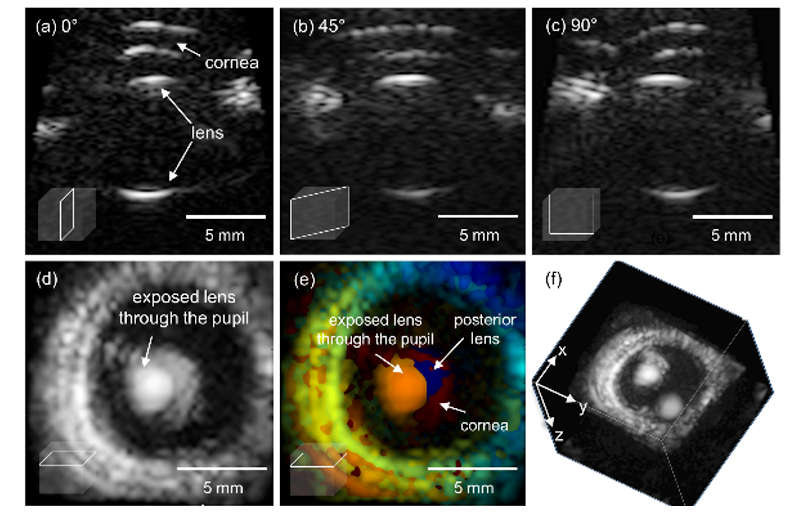
FIG. (a-c) Multi-planar imaging capabilities; real-time (d-e) C-scan and (f) Volumetric imaging
PHOTOACOUSTIC IMAGING
Photoacoustic imaging is a promising and emerging biomedical imaging modality that provides optical contrast at relatively deep depth complemented to traditional ultrasound imaging. This technique is based on the photoacoustic effect where ultrasound waves are generated due to light absorption in the tissue’s chromophores. Therefore, photoacoustic imaging is capable providing non-invasive, real-time images of structural information with physiological functional features, such as oxygen saturation, representing hypoxia or the progression of cancer invasion and metastasis, and cellular and molecular signatures of tissue with the assist of exogenous photoacoustic contrast. Over the past few years, the technology has been extensively applied to numerous human diseases. Diagnosis in early stage and treatment of various diseases can be screened and monitored by using photoacoustic imaging with endogenous chromophores. In addition, novel approaches using nano photoacoustic contrast agents have been also significantly investigated to improve for enhanced contrast, targeting and therapeutics over traditional probes for molecular theranostics.
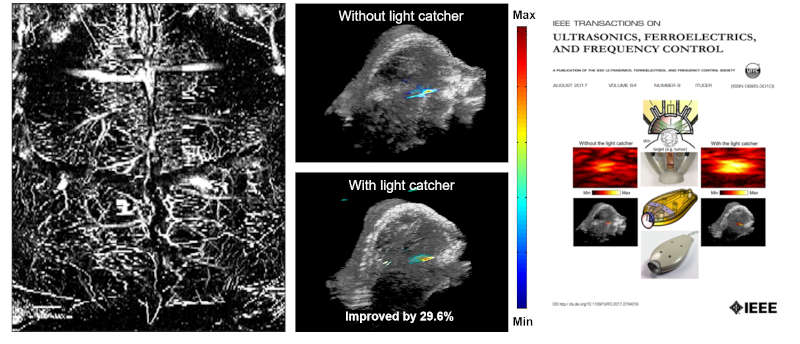
FIG. Mouse brain imaging (Image courtesy of Chonnam University, Dr. Changho Lee), Enhanced light delivery scheme for Photoacoustic imaging (Featured on the cover of IEEE Transactions on UFFC, Aug, 2017)
NANOTECHNOLOGIES FOR MOLECULAR PHOTOACOUSTIC IMAGING
Photoacoustic effect refers to the generation of acoustic waves by the excitation of optical radiation. Several mechanisms for generating photoacoustic signals have been known, which include electrostriction, thermal expansion, photochemical changes, gas evolution and plasma formation. For its reasonable photoacoustic conversion efficiency and safety, photoacoustic agents based on thermal expansion have been traditionally and widely utilized in biomedical imaging applications. In recent years, optically-triggered phase-transition droplets were introduced as a promising photoacoustic contrast agent that produce significantly stronger photoacoustic signals through the vaporization process in response to a short pulse laser. If synthesized in nanoscale, these tiny droplets before being activated and vaporized can passively accumulate to the target area in a tumor, due to the enhanced-permeability and retention effect. These droplets can also be utilized as a dual-mode contrast agent for combined ultrasound and photoacoustic multi-modal imaging, as the laser-activated droplets that become gaseous microbubbles are hyperechoic due to significant mismatch of acoustic impedance at the boundary.

FIG. Perfluorocarbon-based phase-transition droplet for Photoacoustic imaging
Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury.
Kidney International, 2020 , ,Vol. 0No. 0 ,pp. 0 ~ 0(SCIE, 0:0.0
[Q. Chen, J. Yu], L. Lukashova, J D. Latoche, J Zhu, L. Lavery, K. Verdelis, C J. Anderson, and K. Kim
Validation of ultrasound super-resolution imaging of vasa vasorum in rabbit atherosclerotic plaques
IEEE Transactions on UFFC, 2020 , ,Vol. 0No. 0 ,pp. 0 ~ 0(SCIE, 0:0.0
J. Yu, H. Yoon, Y. Khalifa and S. Emelianov
Design of a Volumetric Imaging Sequence Using a Vantage-256 Ultrasound Research Platform Multiplexed with a 1024-Element Fully-Sampled Matrix Array
IEEE Transactions on UFFC, 2019 , ,Vol. 0No. 0 ,pp. 0 ~ 0(SCIE, 0:0.0
















